Good Documentation Practices (GDocP)
Created By Ariyono W Ardi , Drs. Apt. MM .On 24 Oct 2022
Good Documentation Practice (GDocP) is a systematic procedure from preparation, review, approval, issuance, recording, storage and archiving of documents.
What is Good Doc Practice (GdocP)….?
- Documents are information (meaningful data) and their supporting media, in the form of paper, CDs, computer files, microfilms, x-ray films, etc.
- Documents provide information or evidence or can serve as official records.
- Record is a document that states the results achieved or provides evidence of the activities carried out.
- A Guideline is a document that describes recommended practices and instructions.
- Policy (Policy) is an adopted plan or action or principle of action which is intended to influence and determine the decisions or actions of the organization.
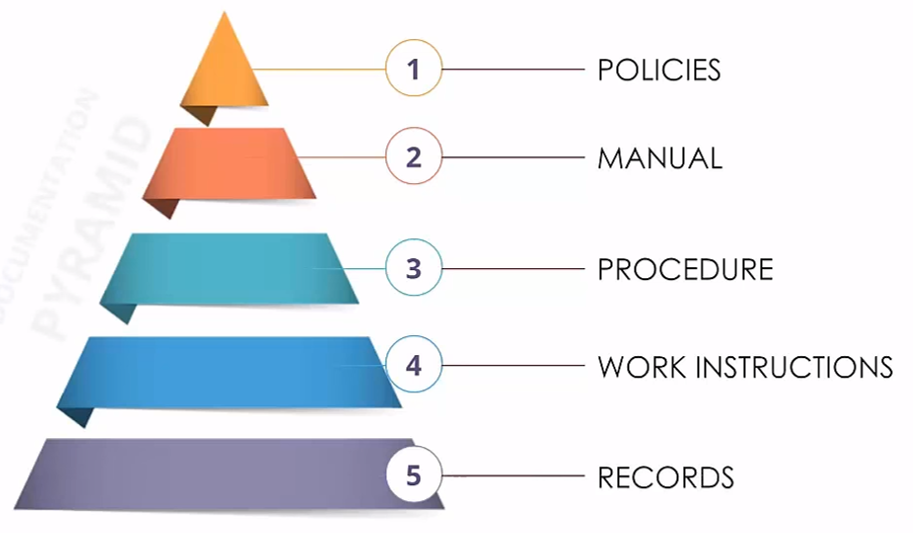
Documents Pyramid

Why Is GdocP So Important To Customers?
- Some customers are companies that apply strict quality management rules.
- Documentation errors can lead to critical consequences related to:
- Product safety
- Court proceedings
- Violation of government rules
- Good Documentation Practices (GDocP) is required in terms of ensuring correctness of actions taken vs. SOPs as evidence on when auditing
- GDocP applies to all processes documentation included in the quality management system which is continuously being developed
- GMP is part of quality assurance which ensures that products are consistently produced and controlled to the appropriate quality standards for their intended use.
- Documentation is also key to GMP compliance as it ensures traceability of all activities ranging from development, production processes, and testing.
- Documentation is a means for auditors to assess the overall quality of operations within the company and the final product.
- Documentation is also the key to CPO compliance. B because it ensures traceability of all activities from development, production processes, and testing
GDocP Principles
- Documents with original signatures should not be destroyed.
- Never falsify information
- Don't use Tip-ex to replace mistakes
- Never erase information or notes
- Don't use a pencil to fill in data/information
- All information must be written in permanent Black or Blue ink
- In the document/record no spaces/columns are left blank.
- Never use symbols such as ditto marks or arrows to indicate successive repetitions of activities.
- Computer prints taken on thermal paper or ink that fade easily should be photocopied and maintained. Originals must be destroyed after verification
- GMP requires that documents should be:
- Controlled within the framework of the Quality Management System
- Approved, signed and dated
- Periodically must be reviewed
- Retained to a certain time, and
- Can be updated within the framework of the Quality Management System.
- Don't:
- Don't delete any pages or sections from the log book.
- Do not take temporary notes on scraps of paper or in the palm of your hand
- Keep notebooks intact
- Do not take notes in post-it®
- Do not copy data
- Avoid using unregistered laboratory notebooks
- Re-writing or rewriting notes should be avoided
- If necessary, do the following :
- Approval from the relevant supervisor and QA
- Document identification by “re-write”
- Notes may be rewritten, only if:
- Original notes can be read clearly
- Incorrect form or document used
- records can be corrected
- the original notes are in a format that cannot be stored for long (thermal) papers).
- If necessary, do the following :
- Witnessed/Checked/Reviewed by
- When one person performs a task and a second person is in charge of verifying that the task was performed correctly
- Double checking provides additional assurance that no errors have been made
- The person performing the verification must be clear what they are verifying ->their signature and date on document
Types of Documents
- Specifications
- Processing and packaging instructions
- Standard operating procedures (SOP)
- Records related to quality.
- Technical agreements
- Confidentiality agreements
- Technical reports
- Other documents related to Quality Quality Management
- Deviation reports
- Audit plans
- Validation Master Plans and validation documents including URS, DQ, FAT, IQ, OQ, PQ, and Validation reports
- Material related documents The test includes product specifications, acceptance of test materials, and test results reports.
- Documents related to employees, such as: employee training and health records.
- Personnel related documents including training records
- Change control
- Worksheets, notebooks, and logbooks
- Quality Manual
- SOP's
- Validation protocols and reports
- Facilities related documents, eg floor, HVAC and environmental specifications.
- Deviation Form for both unplanned deviations and system failure investigation
- Change control
- Worksheets, notebooks, and logbooks
- Quality Manual
- SOP's
- Validation protocols and reports
Characteristics of GDocP
The 8 Rules
- Permanent
- Information cannot be changed, erased, or erased
- Correct stationery (GMP)
- Not allowed to use pencils
- Ink permanent
- Black or Blue
- Provides a consistent look and standard of the documen
- Legible
- Information can be easily read
- If there is an error, correct it correctly
- over the original
- Do not do:
- Strike out or black out with a pen
- White out
- Write over
- Explain the reason for the error
- Write the correct information correct
- Initials and dates
- Factors to consider:
- legibility of numbers and letters character
- 0 and 6
- U and V
- 1 and 7
- 3 and 8
- B and 8
- Information can be easily read
- Accurate
- Calculations are correct and information is recorded carefully
- Perform calculations at least Twice Have
- calculations reviewed and verified by a second person
- Present results in predetermined units specified
- Product name writing is correct
- Lot number, serial number, and product code are double checked
- Calculations are correct and information is recorded carefully
- Prompt
- Information is recorded in a timely manner. Actions are documented immediately, never before.
- Document activities immediately after they are performed Never do documentation before or after the date!
- If you miss a step in a process, be honest. Document and complete the step at that time if possible, noting the actual date when the activity was completed.
- Consistent
- Complete
-
- All information included
- No blank space, page, or page section
- N/A can only be used if it is very clear that part of the document, form, record is invalid.
- Provide an explanation with N/A (Example “N/A” due to repeated tests” Initials and Date.
- For unused tables, Draw a line (“/”) on unused tables, explain with N/A, then initial and give the date.
- Draw a “Z” line on the blank page and write “blank”, initial and date
- All information included
- Direct
-
- Information is recorded immediately into the appropriate form, laboratory notebook or computer system.
- Original data is entered directly into the GMP document.
- No there are notes on used paper and or post it (sticky paper).
- Do not write on raw material containers, cardboard, gloves, etc
- Information is recorded immediately into the appropriate form, laboratory notebook or computer system.
- True to reality (Truthful)
- The correct information contained in the document is factual.
- Your signature/initials say that the information is True
- The information you report must be factual Information should not be falsified
- The correct information contained in the document is factual.
Characteristics of GDocP
Examples of GDocP Implementation Date
- format date
- Documentation at the time the activity was carried out
- Any changes are documented
- Deviations are immediately caught Corrections
- are recorded effectively and accurately
- Empty areas are identified correctly and with a documented explanation.
Common Errors in Documentation
- No signature and date at the time the activity was performed.
- Write-over
- Date and signature writing are not uniform.
- Blank space
- Unreadable writing
- Too many corrections
- Documentation not done immediately
- Use of the words “idem/dito” on the same job
- Using signature stamp
- Failure to use ink as prescribed by procedure
- Logbook correction failed to identify the person make changes
- Original data that is not clearly legible
- Use of pencils
- Inaccurate records
- Sample order tables and audit trails are not documented.
- Undated handwriting changes
- Double writing, multiple correction lines, and use of Tip-Ex or other disguise tools.
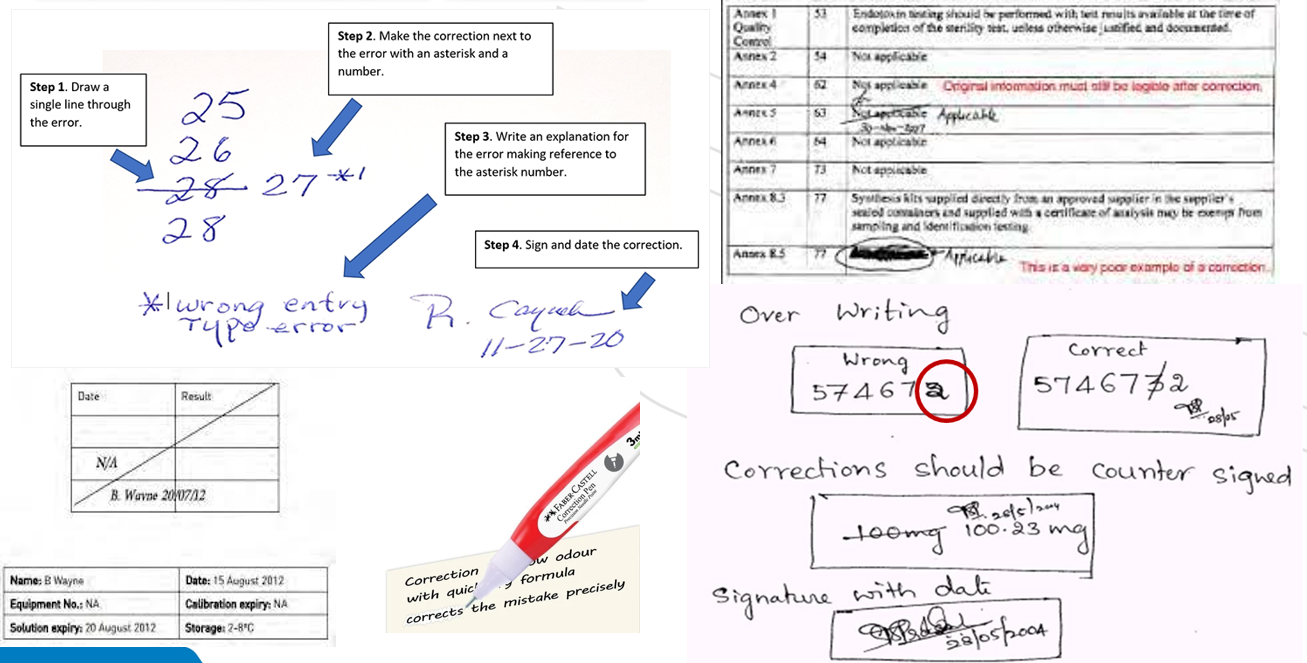
- Correction of documentation errors should include:
- Cross out with one line above the wrong information
- Write corrections next to errors
- Write an explanation of why the error occurred
- Initial and date of corrections
Documents Management
- Every pharmaceutical organization should have a system for documentation management, which contains rules and mechanisms for Create and control documents
- Assign someone to be responsible for controlling the documentation system
- Ensure layout, approval, authorization, and unique identification of all documents related to quality controlled in Master SOP
- Have Master Documentation SOP including :
- Procedures for issue (issue), withdrawal, reissue, document maintenance and traceability
- Document identification system including batch manufacturing record
- Document linkage with government regulations or other cGMP rules.
- A special audit is required for system management documentation.
- Guarantee that only the latest version of the document is used in the field.
- storage and archiving time
- Document Control
- must be available at every point of use
- Master, including electronic versions, must be controlled
- There is control over document format
- There is a system for monitoring changes, approvals, and reissues
- There is control over documents originating outside
- Document Creation
- Documents must be up to date with the events they describe
- Documents must not be handwritten (except data/information which must be filled out manually)
- When produced electronically, documentation must be checked for accuracy
- Error free
- For some types of data, documentation must be in a format that allows for evaluation trend data.
- Document Approval
- Documents must be approved for use. Documents must be approved, signed and dated by authorized personnel.
- Hand written entry
- Adequate space needs to be provided for handwritten entry.
- Filling in by hand must be with indelible ink.
- Filling in critical data must be checked independently (second person verified).
- There is no space for handwritten entries to be left blank. If space is not used, the blank space should be crossed out or "n/a" (or similar text). Writing “Ditto” is not acceptable
- Stamp in lieu of a handwritten signature is not acceptable
- Document Copies
- Copies must be clear and legible
- Errors are not allowed
- Documents must be reviewed regularly and kept up to date
- Documents must be retained and available for audit
- Documents in archives must be available retrieved for an appropriate duration
- Electronic document management systems must be validated
- Electronic records must be duplicated as “Back up”
- Document Modification
- Handwritten modifications signed and dated
- Changed text should not be obscured
- If necessary, reasons for changes should be recorded
- Controls to prevent unauthorized use of old documents Accidental
- Electronic Record
- We can use the laboratory information management system (LIMS) to record test results electronically
- As a controlled form created in Microsoft Word (electronically) it should be printed on paper for inspection so that it can be signed handle for approval
- Document Storage
- Important records must be stored in a secure place, with limited access to authorized persons.
- The storage location must ensure adequate protection from loss, destruction, or falsification, and from damage from fire, water, and other disasters.
- Records important for regulatory compliance or to support critical business activities must be reproduced on paper, microfilm or electronic, and kept in a separate secure location in a separate building from the original
Observation in Poor GDocP
- No signature and date of activity done
- Dates and signatures are not uniform
- Activities carried out on one day and signing on another Day
- Blank space
- Unreadable writing
- Too many corrections
- Overlapping writing and repeated correction lines
- Use of eraser device
- Use of pencil
- Error correction not signed and dated, and reason correction not written
- Untraceable
- Data integrity is questionable