We help to align process validation activities with a product life cycle concept and with existing FDA Guideline including ICH-Q08 (Pharmaceutical Dev), ICH-Q09 (Quality Risk Management) & ICH-Q10 (Pharmaceutical Quality System).
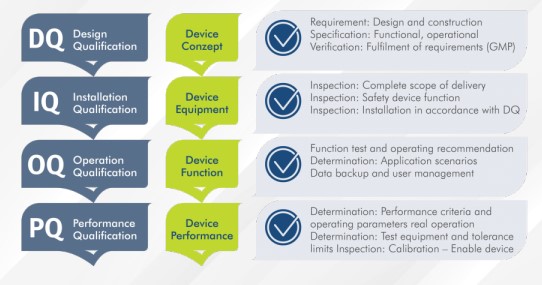
This service also covers support for developing Validation Master Plan (VMP) and other related documents based on the risk based analysis and guidance for implementing Design Qualification(DQ), Installation Qualification (IQ) and Operational Qualification (OQ) including review and guidance for execution and record keeping. This will ensure that validation is implemented smoothly and without redundancy.